4 Chemical pollution of water
4.1 Levels of toxic substances
Toxic substances are a feature of the natural world; many plants contain chemical compounds that make them anything from mildly distasteful to lethally poisonous to animals that might eat them. Some animals are equipped to deal with dangerous plants in their environment and possess detoxification mechanisms that break down harmful compounds. Humans, for example, rely on their liver to neutralise the harmful effects of alcohol. During human cultural evolution, cooking techniques have developed that destroy toxic chemicals in plants. Examples include nerve poisons in the lentils from which dahl is made in India, and cyanide in cassava, a staple crop in Africa and South America. There are limits, however, to what the liver and cooking can achieve and the environment contains a huge array of chemical compounds, some of them of human manufacture, that are harmful to human health and survival. The latter are said to be xenobiotic (zen-oh-bye-ot-ik). Literally meaning ‘alien to nature’, in the context of this unit this word refers to chemicals ‘of human origin’.
Water is said to be polluted, or contaminated, whenever any harmful or undesirable change in its physical, chemical or biological quality results from the release into it of synthetic or naturally occurring chemicals, radioactivity or organic matter. (Organic means arising from the bodies of plants, animals or other organisms.) Pollution often refers to the results of human activity but there are significant natural causes of contamination, such as volcanic eruptions, which release a variety of chemicals, and tsunamis, which mix salty seawater with freshwater. Much of the groundwater obtained from boreholes in parts of Bangladesh and West Bengal is contaminated with naturally occurring arsenic, released from rocks deep underground.
Contamination can occur at many points in the global water cycle depicted in Figure 3. Most familiarly, pollutants can be released into rivers or into the sea, but they can also be released into groundwater by pollution of the soil. Some pollutants enter the water cycle from the atmosphere; for example, acid rain is caused by the mixing of water vapour with gaseous pollutants such as sulfur dioxide, released by burning fossil fuels, and a variety of nitrogen compounds from agricultural fertilisers (see Section 4.4).
Pollution may be acute or chronic. Acute pollution events refer to the sudden release of large quantities of a contaminant, usually leading to very obvious harmful effects. An example is provided by the accidental release of a large quantity of aluminium sulfate, a substance used in water treatment, into the water supply of Camelford, in Cornwall in July 1988, leading to severe loss of mental function in a large number of people (Altmann et al., 1999). Chronic pollution refers to the slow and persistent contamination of water through the sustained release of a pollutant and is, in many ways, a more serious concern, for three reasons. Chronic pollution may go undetected for a long time; it is generally more difficult to rectify than an acute pollution event; chronic pollution is also serious because, unlike most acute pollution events, it is often not confined, as the Camelford tragedy was, to a small area.
One of the most toxic of xenobiotic pollutants, dioxin, formed by the burning of plastics and certain fertilisers, has become ubiquitous (found everywhere) and can be detected in virtually every person that has been tested for it throughout the world (Sargent, 2005).
As a result of the risks arising from pollution, the water supply in high-income countries is carefully monitored to ensure that levels of contaminants do not exceed specified concentrations that are considered to be safe. The determination of safe levels is quite a complex process that involves the science of toxicology (the study of toxins and their effects on living organisms). This involves exposing animals to a toxic compound to determine its lethal dose. Among the animals used for toxicological testing of water-borne chemicals are tadpoles of the African clawed frog (Xenopus laevis) (Figure 7). Xenopus is widely used for this purpose as it breeds readily in captivity and produces huge numbers of tadpoles. Frogs and amphibians are regarded as particularly sensitive indicators of environmental damage.

There is growing recognition that determining the lethal dose of a compound in Xenopus tadpoles in a laboratory is not very helpful in trying to determine if that compound poses a threat to human health.
SAQ 11
Why do you suppose this is?
Answer
For a start, there is the question of whether Xenopus is more or less sensitive to the compound than humans. Furthermore, the lethal dose approach is a measurement only of mortality, and provides no information about morbidity.
Another approach to the study of pollution is the science of ecotoxicology, which is the study of the fate of contaminants in the natural environment and of their effects on plants, animals and ecosystems. (Ecosystems are recognisable assemblages of plants and animals, such as woodland, grassland, rivers, etc., in which a distinct set of plants and animals live together and interact with one another.) This asks, not how much of a compound does it take to kill a tadpole, but what is the effect on a tadpole of the compound at the kind of concentration that occurs in the wild?
Much environmental damage has been done in Scandinavia by acid rain caused by industrial pollution from the industrial north of Britain, swept across the North Sea by the prevailing wind. For example, populations of frogs and newts have declined widely and disappeared altogether in some localities. If frog tadpoles are reared in water that is acidified to the same level as Scandinavian pond water, they do not die. Rather, they become lethargic and feed less than tadpoles in non-acidified water (Griffiths et al., 1994). They do not grow as fast as healthy tadpoles; they later become under-sized frogs and they do not survive to breed.
The result is the same as if tadpoles dropped dead when exposed to acid water; the frog population declines. By focusing on often very subtle, non-lethal effects of pollutants, ecotoxicologists provide a more complete and precise assessment of how pollutants affect wildlife than can be derived by determining their lethal dose. If it seems odd to you that scientists study the possible human health effects of xenobiotic chemicals by looking at their effects on tadpoles, bear in mind that it would be impossible, for ethical reasons, to test the effects of such chemicals on humans directly.
Bioaccumulation
When chemical contaminants enter the body of a person, they circulate around the body in the blood. Different contaminants have different chemical properties and specific contaminants tend to accumulate in specific parts of the body, called target tissues, or in substances produced in the body such as breast milk (Table 3).
Table 3: Some common pollutants and their target tissues
| Pollutant | Target tissues or substances |
|---|---|
| lead | bone, teeth, nervous tissue |
| mercury | nervous tissue, particularly the brain |
| organochlorine pesticides, polychlorinated biphenyls (PCBs) | fatty tissue, breast milk |
| asbestos | lungs |
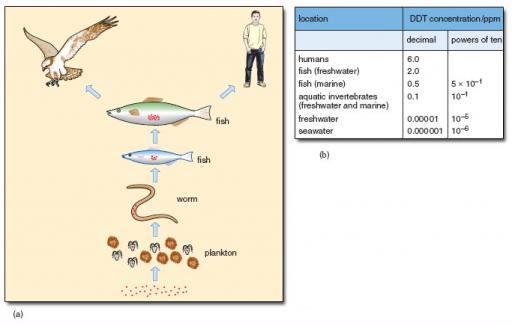
The affinity of specific pollutants for specific target tissues is related to a very important aspect of ecotoxicology, called bioaccumulation. This refers to the fact that, having been released into the environment, a pollutant is not randomly or evenly dispersed, but becomes concentrated into particular components of ecosystems. For example, DDT is accumulated in the fat reserves of birds, where it can reach quite high levels. (DDT, dichloro-diphenyl-trichloroethane, was the first widely used synthetic pesticide and has been used to kill agricultural and domestic insect pests since 1939; see Section 4.2.) This has two important effects. In the affected bird it means that, if it uses its fat reserves to provide energy for some specific activity, such as reproduction or migration, a large dose of DDT is released into its blood over a short time. Every time a predator eats such a bird, it too receives a large dose which, in turn, is stored in its fat. The consequence of bioaccumulation is that contaminants that may be quite safe to wildlife, or humans, when encountered at the kind of concentrations at which they are released into water, can become concentrated at particular points in the food-chain at levels that are not safe (Figure 8).
Original Copyright © 2007 The Open University. Now made available within the Creative Commons framework under the CC Attribution – Non-commercial licence (see http://creativecommons.org/by-nc-sa/2.0/uk/).